
How to Attract Investors for your Medical Device
Do you have a great idea but need the money to fund it? Check out this blog for a step-by-step guide to attracting investors for your medical device start-up.

Navigating the Future of Healthcare: The Role of Medical Device Consulting
Medical device consulting plays a pivotal role in bringing innovative technologies to the forefront. These consultants act as strategic partners, guiding manufacturers, healthcare providers, and regulatory bodies through the complex journey of developing, implementing, and regulating medical devices. This article delves into the significance of medical device consulting, its key functions, and the impact it has on shaping the future of healthcare.

Important Changes Impacting Manufacturers of Products without an Intended Medical Purpose
Specific requirements are outlined for:
contact lenses,
products intended to be totally or partially introduced into the human body through surgically invasive means for the purpose of modifying the anatomy such as breast implants,
dermal or mucous membrane filling, equipment for liposuction, lipolysis or lipoplasty, lasers and intense pulsed light equipment
and brain stimulation devices that apply electrical currents or magnetic or electromagnetic fields that penetrate the cranium to modify neuronal activity.

Quality Assurance and Regulatory Affairs Outsourcing
A growing number of small to medium-sized medical device companies are realizing that using highly experienced third-party consultants or specialists, such as those individuals at Methodize Inc., to take care of regulatory and quality compliance saves time and money.

A Comprehensive Guide to Technical File and Design Dossier Development in the Medical Device Industry
In the dynamic landscape of the medical device industry, the development of a robust Technical File (TF) or Design Dossier (DD) is critical for regulatory compliance and successful market entry. This comprehensive guide explores the key aspects and best practices involved in creating a thorough technical documentation.

Demystifying FDA 513(g): A Guide to Pre-submission Interaction with the FDA
FDA 513(g) refers to a section of the Food, Drug, and Cosmetic Act that allows device manufacturers to request information from the FDA regarding the regulatory status of a device. The primary objective is to gain clarification on the applicable regulatory requirements and determine the most appropriate pathway for bringing a medical device to market. This mechanism is particularly valuable in situations where there is uncertainty or ambiguity about the classification or regulatory requirements of a specific device.
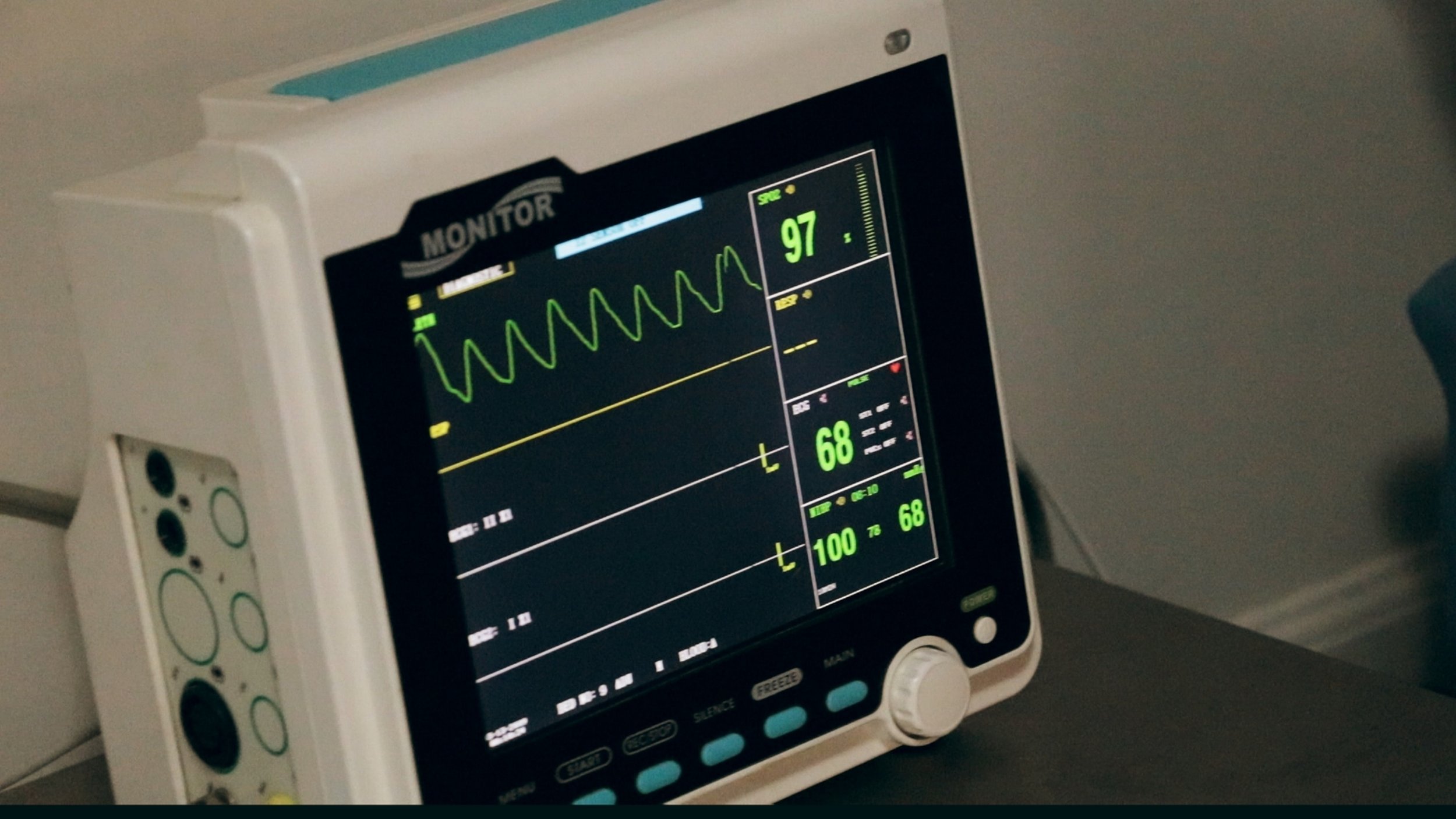
Navigating Quality Standards: A Comprehensive Guide to ISO 13485 Training and Consulting
Navigating Quality Standards: A Comprehensive Guide to ISO 13485 Training and Consulting

Mastering MDR Technical Documentation: A Comprehensive Guide
Medical Device Regulation (MDR) technical documentation plays a pivotal role in ensuring the safety, efficacy, and compliance of medical devices in the European Union (EU). As of my last knowledge update in January 2022, the EU MDR has introduced significant changes, placing a greater emphasis on comprehensive and transparent technical documentation. This article delves into the key aspects of MDR technical documentation, providing insights for manufacturers navigating the complex regulatory landscape.
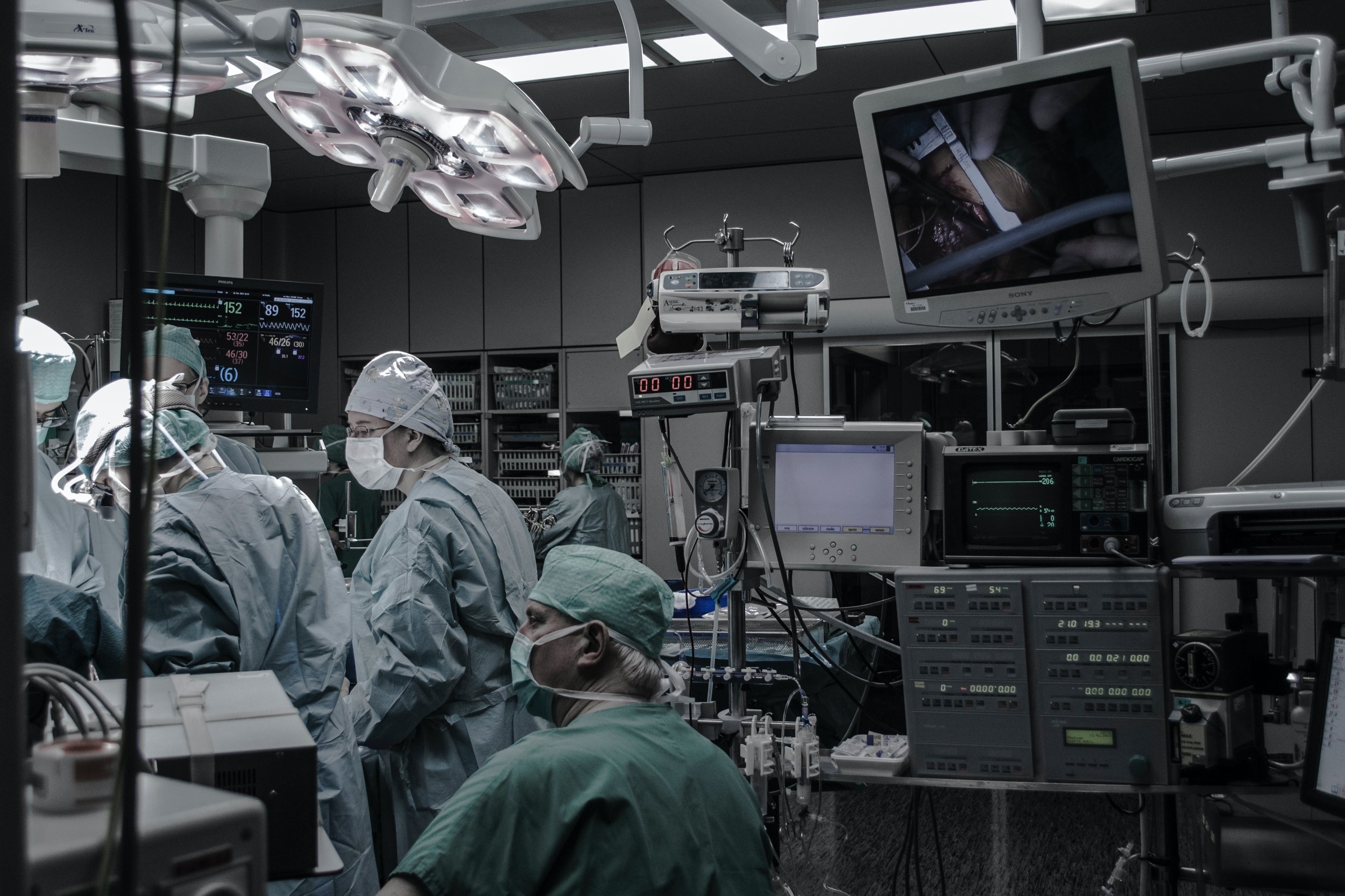
Unlocking Success: The Key Benefits of Medical Device Consultants
In the ever-evolving landscape of healthcare, medical device companies face numerous challenges ranging from regulatory compliance to technological advancements. To navigate this complex terrain and ensure the success of their products, an increasing number of companies are turning to medical device consultants. These experts bring a wealth of knowledge and experience to the table, offering invaluable support in areas critical to the development, approval, and market access of medical devices.

